Abstract
Background: Venetoclax (VEN) is an oral, small molecule inhibitor of the anti-apoptotic protein BCL-2, which may mediate the viability and chemoresistance of acute myeloid leukemia (AML) cells. VEN in combination with hypomethylating agents (HMAs), given its promising efficacy, may provide a therapeutic option for patients with treatment-naïve AML who are not candidates for standard induction therapy.
Methods: This dual-stage (dose escalation and expansion), open-label, phase 1b study (NCT02203773) is being conducted to determine the safety and preliminary efficacy of VEN in combination with decitabine (DEC) or azacitidine (AZA). Eligible patients included those ≥65 years of age with previously untreated AML (defined by WHO criteria) who were ineligible for intensive chemotherapy. Exclusion criteria were previous receipt of HMAs or chemotherapy for antecedent hematologic disorder, favorable risk cytogenetics, known CNS involvement, and WBC count >25 × 109/L (hydroxyurea use permitted). Treatment consisted of oral VEN, which was administered with a short ramp-up during cycle 1 from 20 mg (escalation stage) or 100 mg (expansion stage) to a final dose of 400, 800, or 1200 mg daily. VEN was administered with either DEC 20 mg/m2 given intravenously (IV) on days 1-5 or AZA 75 mg/m2 given IV or subcutaneously on days 1-7 every 28 days (per cycle). Adverse events (AEs) were monitored for safety. The objective response rate (ORR, including complete remission [CR], CR with incomplete blood count recovery [CRi], partial remission [PR], and morphologic leukemia-free state [MLFS]) and overall survival (OS) were evaluated.
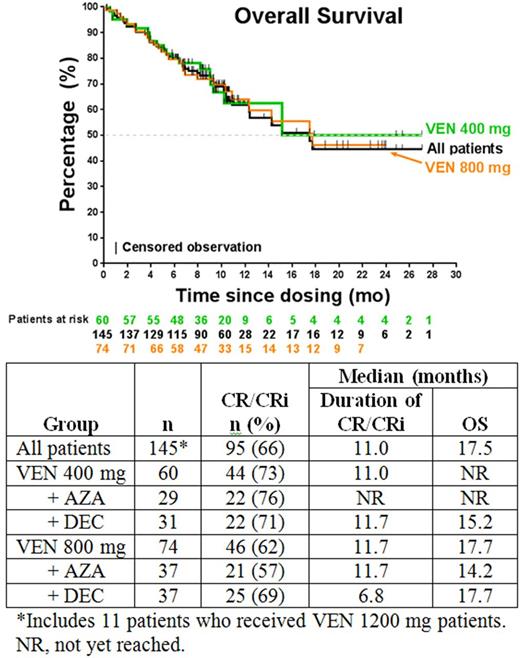
Results: The data cutoff date was February 17, 2017. A total of 145 patients were enrolled (81 male [56%]; median age, 74 years [range, 65-86 years]): 60 received VEN 400 mg (29 with AZA, 31 with DEC), 74 received VEN 800 mg (37 each with either AZA or DEC), and 11 received VEN 1200 mg (5 with AZA, 6 with DEC). Cytogenetic risk was intermediate in 74 patients (51%) and poor in 71 (49%), and 36 (25%) had secondary AML (after myelodysplastic syndrome/myeloproliferative neoplasm or prior chemotherapy). AEs possibly related to VEN (occurring in ≥20% of patients) were nausea (43%), thrombocytopenia (38%), neutropenia (34%, n=49), decreased WBC count (26%). Grade ≥3 AEs possibly related to VEN (in ≥10% of patients) were neutropenia (34%), thrombocytopenia (33%), decreased WBC count (26%), anemia (13%), and febrile neutropenia (13%). SAEs possibly related to VEN (in n≥2 patients) were bacteremia or sepsis (n=8) and pneumonia (n=5). Five deaths occurred ≤30 days after the first dose of study drug; causes were sepsis (n=2), bacteremia (n=1), respiratory failure (n=1), or multiorgan dysfunction (n=1). Eleven deaths total (8%) occurred at ≤60 days; causes in addition to those occurring at ≤30 days were progressive disease (n=4), respiratory failure (n=1), and sepsis (n=1). Seven patients who discontinued the study before first disease assessment were not evaluable, and 1 patient is in active treatment and has not reached first assessment. Overall and subgroup response rates are shown in the Table. Response rates in patients with intermediate- and poor-risk cytogenetics, respectively, were CR 41% and 30%, CRi 34% and 27%, PR 1% and 1%, and MLFS 14% and 18%. The overall leukemia response rate (CR + CRi + PR + MLFS) for the intent-to-treat population (N=145) was 83%, including 35% CR, 31% CRi, 1% PR, and 16% MLFS.Responses in secondary AML patients were 33% CR, 32% CRi, 2% PR, and 15% MLFS. At a median time on study of 7 months (range, <1 to 27 months), the median OS in all patients (N=145) was 17.5 months (95% CI, 12.3 months to upper limit not reached). Analyses of duration of CR/CRi and OS between the AZA and DEC subgroups and between the VEN 400- and 800-mg subgroups are ongoing, and the preliminary results are shown (Table, Figure).
Conclusions: The combination of VEN with DEC or AZA demonstrated promising efficacy and a tolerable safety profile in elderly patients with AML. The ORR (CR + CRi + PR) was 67% (97/145), and the leukemia response rate (CR + CRi + PR + MLFS) was 83% (120/145). The emerging clinical and exposure response data demonstrated that 400-mg VEN has the best benefit-risk profile. A phase 3 study of VEN 400 mg combined with AZA is underway.
DiNardo: Agios: Honoraria, Research Funding; Daiichi-Sankyo: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding. Pollyea: Takeda, Ariad, Alexion, Celgene, Pfizer, Pharmacyclics, Gilead, Jazz, Servier, Curis: Membership on an entity's Board of Directors or advisory committees; Agios, Pfizer: Research Funding. Jonas: AbbVie, Celgene, Daiichi Sankyo, Pharmacyclics, Genentech/Roche, Glycomimetics, Esanex, Kalobios: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees; Rigel: Consultancy. Wei: AbbVie, Celgene, Novartis, Amgen, Servier: Membership on an entity's Board of Directors or advisory committees; AbbVie, Celgene, Servier: Research Funding; AbbVie, Celgene, Novartis, Amgen, Servier: Honoraria. Kantarjian: Amgen: Research Funding; Bristol-Meyers Squibb: Research Funding; ARIAD: Research Funding; Novartis: Research Funding; Pfizer: Research Funding; Delta-Fly Pharma: Research Funding. Recher: Celgene, Sunesis, Amgen, Novartis: Research Funding; Novartis, Celgene, Jazz, Sunesis, Amgen: Consultancy. Seymour: Celgene, Roche, Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie, Genentech: Research Funding. Dunbar: AbbVie: Employment, Equity Ownership. Xu: AbbVie: Employment, Equity Ownership. Mabry: AbbVie: Employment, Equity Ownership. Potluri: AbbVie: Employment, Equity Ownership. Letai: AbbVie, AstraZeneca, Novartis: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal